Опубликована статья: Orel V.B., Vitkovskaya N.M., Bobkov A.S., Semenova N.V., Schmidt E.Yu., Trofimov B.A. Aldol condensation versus superbase-catalyzed addition of ketones to acetylenes: a quantum-chemical and experimental study // Journal of Organic Chemistry. – 2021. – V. 86. – Iss. 11. – P. 7439-7449. IF 4,354. Q1 (БАЦКП). 04.06.2021. DOI: 10.1021/acs.joc.1c00388
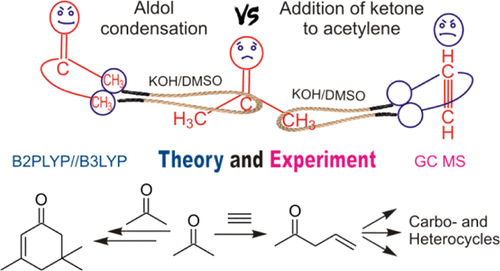
Abstract: The mechanism of aldol condensation of ketones in KOH/DMSO superbasic media has been investigated using the B2PLYP(D2)/6-311+G**//B3LYP/6-31+G* quantum-chemical approach. It is found that the interaction of three ketone molecules resulting in the formation of the cyclohex-2-enone structure [isophorone or 3,5-dicyclohexyl-5-methylspiro(5.5)undec-2-en-1-one] is thermodynamically more favorable than the interaction of two, three, or four molecules of ketone, resulting in the formation of linear products of the condensation. The formation of the condensation products with the isophorone skeleton can significantly hinder the cascade reactions of ketones with acetylenes [to afford 6,8-dioxabicyclo(3.2.1)octanes or acylcyclopentenols] promoted by superbases. In particular, the kinetically more preferable reactions of autovinylation of 2-methyl-3-butyn-2-ol and autocondensation of acetone are the reasons why interaction of acetone with acetylene does not lead to the products of the cascade assemblies. The predominant formation of the products of these side reactions is confirmed experimentally.
Поздравляем авторов статьи и желаем дальнейших успехов!
Пресс-центр ИрИХ СО РАН















